Products
AdvSHENTEK DetectInnova System
The AdvSHENTEK DetectInnova System transformed RMM (rapid microbiological method) into a streamlined, fast, fully automated, and easily portable laboratory system. It integrates nucleic acid extraction and real-time PCR assay, allowing a turn-around time of 2.5 hours from sample to result. Importantly, it only requires less than 5 minutes of hands-on time without specific DNA extraction and PCR expertise to operate.
For Mycoplasma cassette information, please refer to the SHENTEK® AdvSHENTEK Mycoplasma DetectInnova Cassette User Guide (Product No. 1509100).
Easy on-time testing
● Complete automation from sample to result
● Hands-on time < 5 mins
High Sensitivity & Efficiency
● LOD < 10 CFU/mL
● 4 standardized assays in a single run
Accessible Anywhere &Anyone
● No PCR lab or skills required
● Seamless integration from extraction to amplification without manual intervention
Data Reliability
● Fully validated workflow
● Effective internal control across every stage
● 21 CFR Part 11 compliance
High Specificity
● No cross-reactivity with different bacteria or cell lines
● No sample matrix interference
Comprehensive coverage
● Able to detect over 180 species of Mycoplasma, Spiroplasma and Acholeplasma
Performance Characteristics
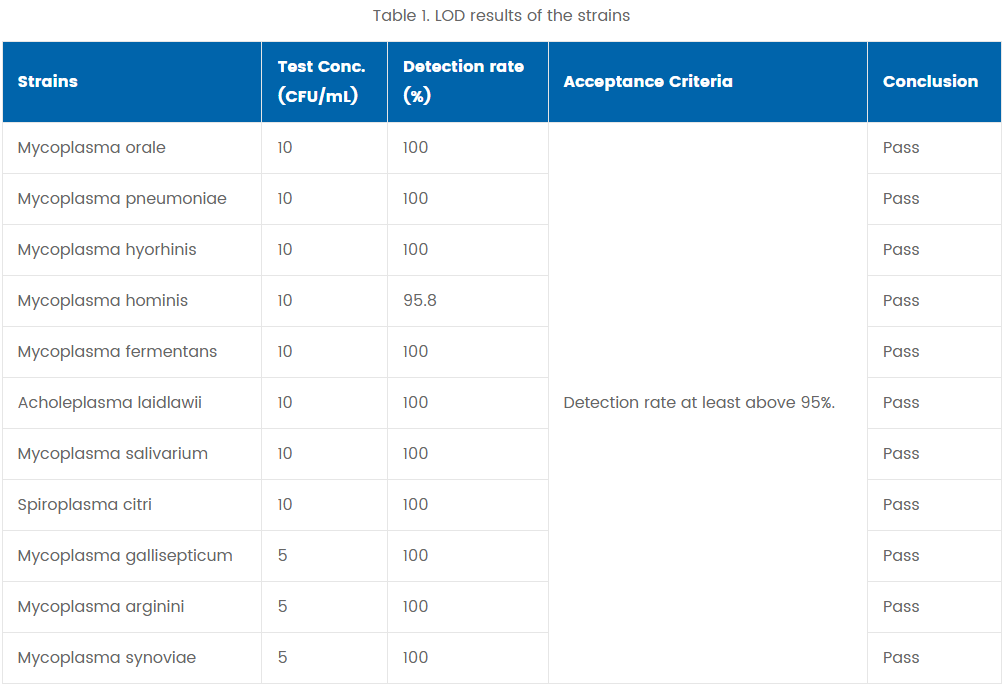
Eight mycoplasma strains at 10 CFU/mL and three mycoplasma strains at 5 CFU/mL were tested. For each strain, 24 assays were conducted and the detection rate was calculated. The results are summarized in the table below.
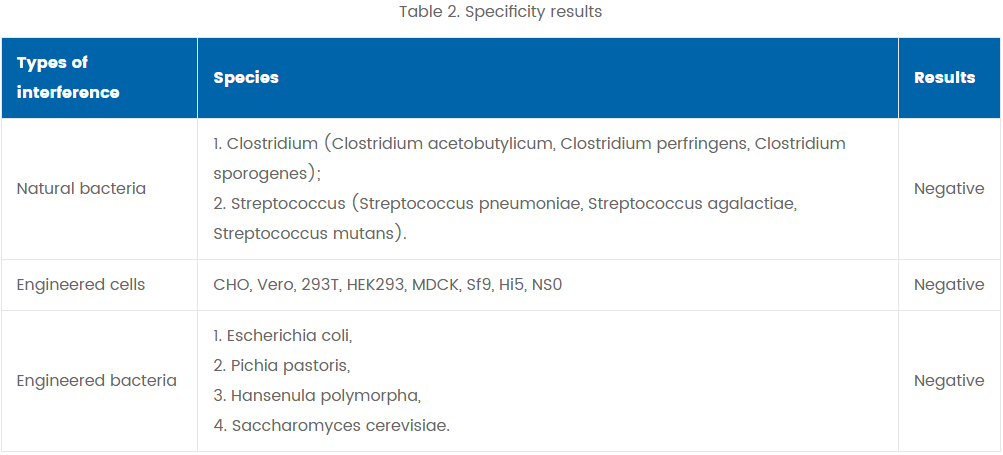
Eighteen genomic DNAs from engineered cells and bacteria, were extracted, purified and tested with the cassette. All test results were negative.
● Instrument precision
Mycoplasma orale 100 CFU was tested on three different instruments by three analysts, with four replicates per instrument. All 12 samples were successfully detected, and both the Ct values of the samples and internal controls met the requirement of CV ≤ 15%.
● Repeatability
Twelve assays were performed on Mycoplasma orale (100 CFU) by a single operator using the same instrument. All tests were successfully detected, and both the Ct values of the samples and the internal controls can meet the requirement of CV ≤ 15%.