Products
HZSKBIO® offers a variety of HCP detection and analysis service platforms:
 HCP assay and analysis platform [ELISA, LC-MS, 2D with different staining]
HCP assay and analysis platform [ELISA, LC-MS, 2D with different staining]
 HCP coverage assessment platform [IMBS-2D, IMBS-MS]
HCP coverage assessment platform [IMBS-2D, IMBS-MS]
 Anti-HCP polyclonal antibody preparation platform
Anti-HCP polyclonal antibody preparation platform
 Customized HCP ELISA kit development platform
Customized HCP ELISA kit development platform
1、HCP Assay and Analysis Service Platform (ELISA, LC-MS and 2D with different staining)
 Process-specific SHENTEK® HCP ELISA kits to ensure accurate and specific detection;
Process-specific SHENTEK® HCP ELISA kits to ensure accurate and specific detection;
 Traceability system for HCP ELISA standards to ensure assay accuracy and reliability;
Traceability system for HCP ELISA standards to ensure assay accuracy and reliability;
 Process-specific and high-risk HCP analysis, as well as lot-to-lot comparison for preclinical and clinical trial lots;
Process-specific and high-risk HCP analysis, as well as lot-to-lot comparison for preclinical and clinical trial lots;
 High-risk HCP identification by applying orthogonal methods to complement the limitations of HCP ELISA for bioprocess development.
High-risk HCP identification by applying orthogonal methods to complement the limitations of HCP ELISA for bioprocess development.
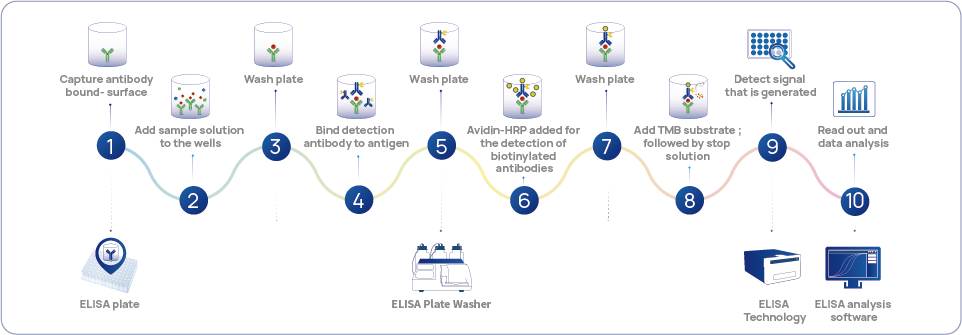
Figure 1 Standard Method for HCP Monitoring - Sandwich ELISA Workflow
2、HCP Coverage Assessment Platform (IMBS-2D, IMBS-MS)
 Proprietary IMBS (immunomagnetic bead separation) technology using antigen-specific antibody immunoaffinity-purification for rapid & specific HCP immunogen isolation;
Proprietary IMBS (immunomagnetic bead separation) technology using antigen-specific antibody immunoaffinity-purification for rapid & specific HCP immunogen isolation;
 Orthogonal proteomic methods of 2D & LC-MS to illustrate antibody coverage of HCPs with increased sensitivity and accuracy;
Orthogonal proteomic methods of 2D & LC-MS to illustrate antibody coverage of HCPs with increased sensitivity and accuracy;
 Antibody coverage analysis on the early process samples or mock samples to select suitable ELISA for the specific bioprocess;
Antibody coverage analysis on the early process samples or mock samples to select suitable ELISA for the specific bioprocess;
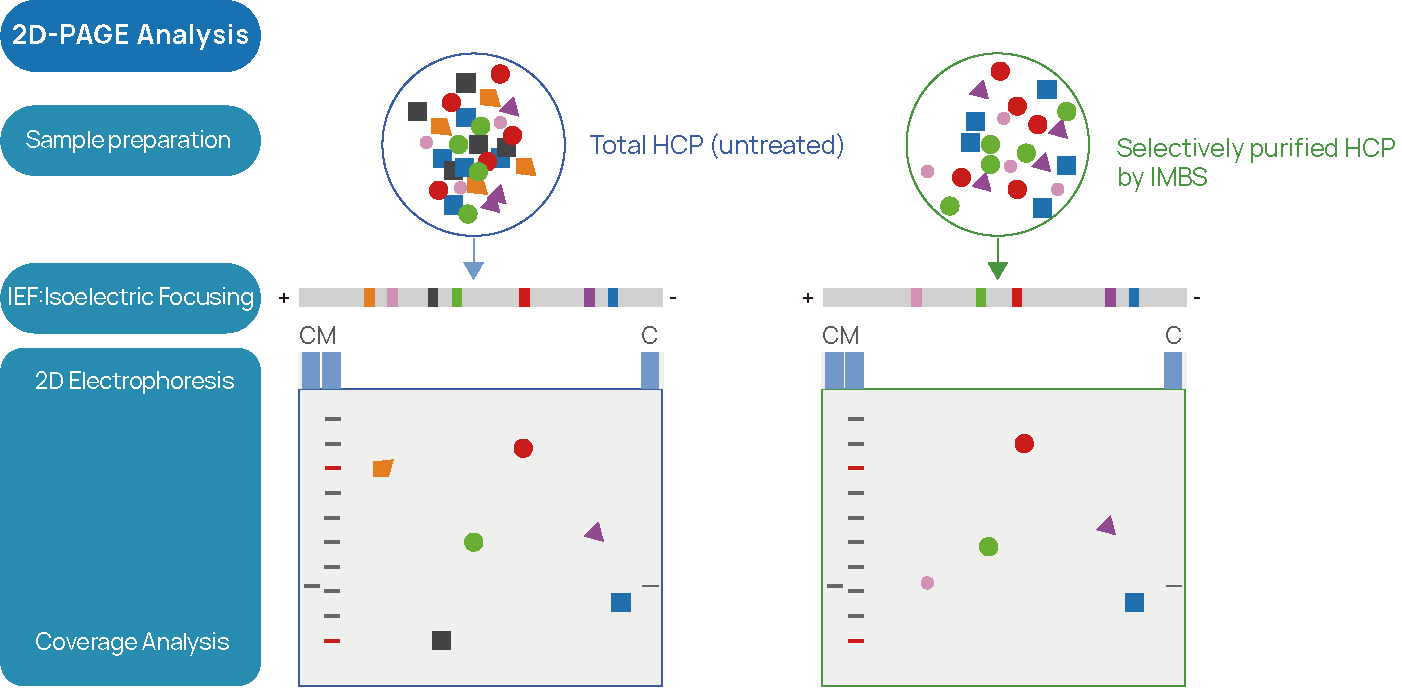
Figure 2 IMBS-2D HCP-antibody Coverage Analysis
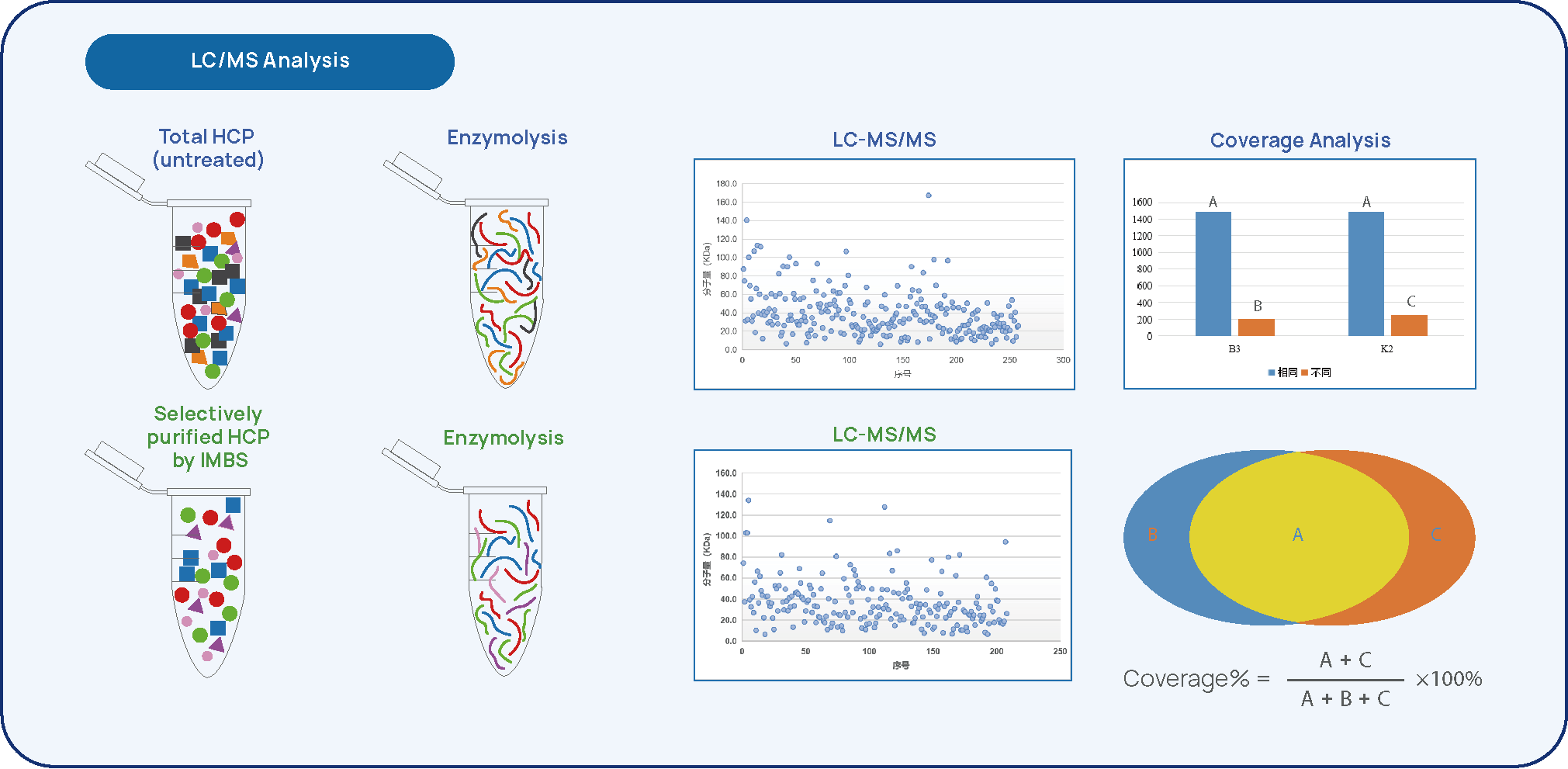
Figure 3 IMBS-LC/MS HCP-antibody Coverage Analysis
3、Anti-HCP Polyclonal Antibody Preparation Platform
 Effective immunization strategies and antibody preparation for different antigen groups;
Effective immunization strategies and antibody preparation for different antigen groups;
 Characterization and quality control of antibodies to maximize quality, coverage, and speed;
Characterization and quality control of antibodies to maximize quality, coverage, and speed;
 Antibody generation, purification and screening with optimized processes;
Antibody generation, purification and screening with optimized processes;
 Standard practice of HCP reference traceability to ensure the reliability of immunoassays;
Standard practice of HCP reference traceability to ensure the reliability of immunoassays;
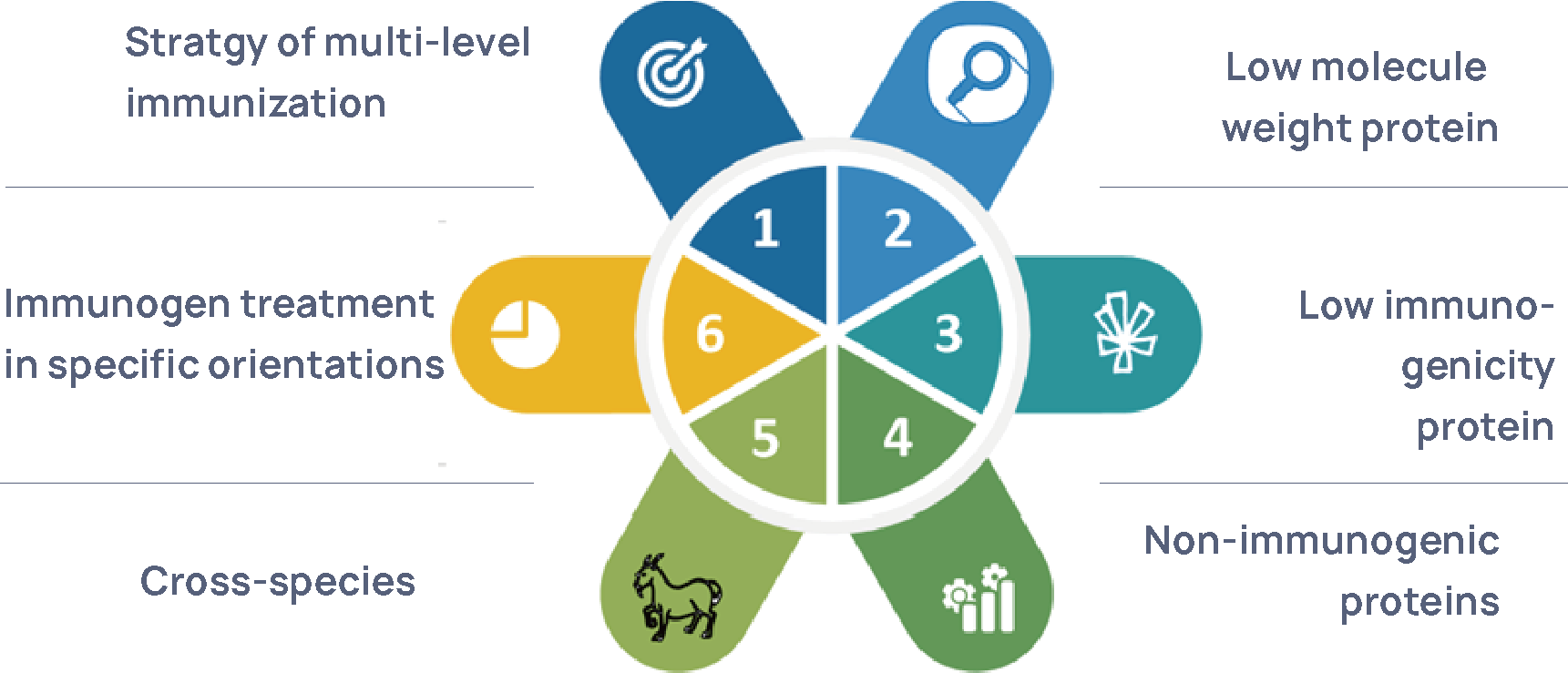
Figure 4 HCP Antigen Preparation and Immunization for Capture Antibody Production
4、Customized HCP ELISA Kit Development Platform
 Comprehensive studies of residual HCP (rHCP) standards to achieve excellent coverage and specificity;
Comprehensive studies of residual HCP (rHCP) standards to achieve excellent coverage and specificity;
 Standard practice of HCP reference traceability to ensure the reliability of immunoassays;
Standard practice of HCP reference traceability to ensure the reliability of immunoassays;
 Effective and intensive antigen and antibody preparation strategies for robust immune response and high-quality polyclonal antibodies;
Effective and intensive antigen and antibody preparation strategies for robust immune response and high-quality polyclonal antibodies;
 Compliance with ISO13485 quality system to guarantee the high-quality HCP ELISA kit development;
Compliance with ISO13485 quality system to guarantee the high-quality HCP ELISA kit development;
 Compliance with CNAS/ISO10725 and GMP quality standards to ensure the integrity of the service and products;
Compliance with CNAS/ISO10725 and GMP quality standards to ensure the integrity of the service and products;
HZSKBIO® Customized HCP ELISA Kit Development Workflow
❶ Assay standard preparation
Comprehensive studies (concentration, composition, stability, traceability, etc.) and production
❷ Antibody preparation
Effective immunization strategies, and antibody tier & coverage validation
❸ Kit Development
Assay set-up and product specification setting
❹ Production Validation
Pilot scale test and process scale-up validation
❺ Method Transfer
Assay validation & transfer test
HZSKBIO® Comprehensive and Customized Residual Host Cell Protein (rHCP) Analysis Service Platforms
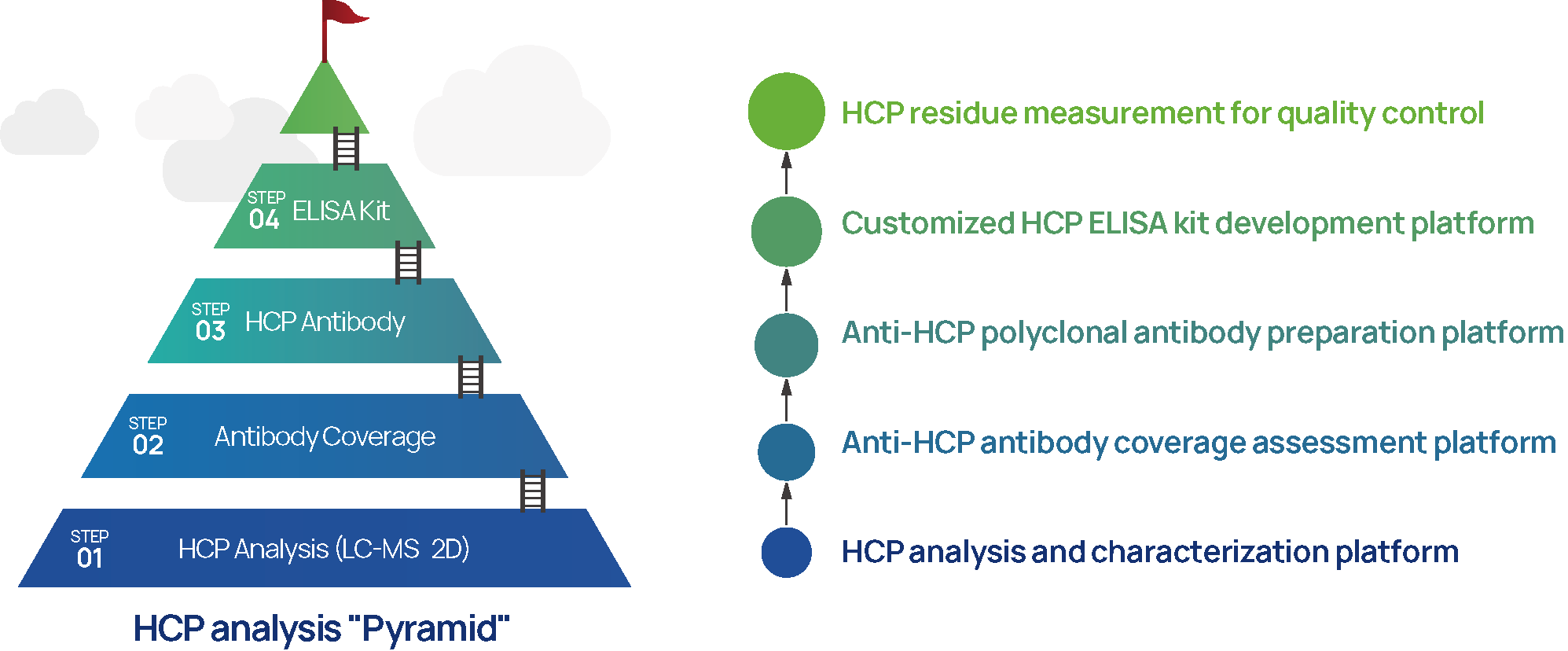
Figure 5 HZSKBIO® Residual Host Cell Protein Analysis Service Platforms
HZSKBIO has established a highly efficient HCP antigen and anti-HCP antibody preparation and analysis platform, which provides customized process or platform-specific HCP reference standard and diverse antibodies, ensuring the efficient development and consistent supply of high-quality HCP ELISA kits.
返回